You may have heard that sparkling water—also known as seltzer or carbonated water—is better for your teeth than soda. While it's undeniable that sugar-free carbonated beverages are less damaging than soda, there still might be some dental issues to think about the next time to reach for that lemon-lime seltzer.
1. The problem with Carbonic Acid
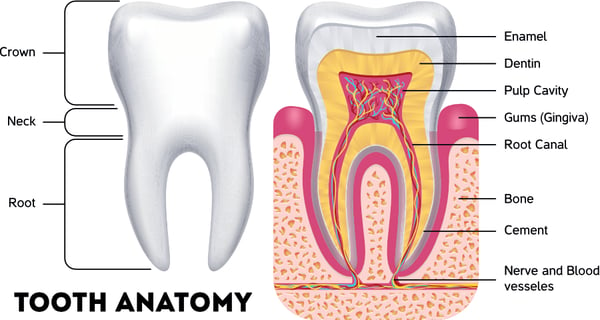
Many people prefer seltzer over plain water because of its pleasant effervescence. That fizzy feeling is caused by carbonic acid, which may not be the best thing for your enamel. Enamel, which forms the hard outer layer of your teeth, is made mostly of minerals like hydroxyapatite.
Seltzers are made with carbonic acid, which can gradually interact with those minerals to wear enamel away over time. On the pH scale, the scientific range from acidity to alkalinity, plain water is a between 6 and 7, towards the center of the scale.
That puts sparkling water at about a 4.5 - 5.5, which is a little more acidic. Although sparkling water is far less potentially dangerous for your teeth than soda, that little increase in acidity has the potential to wear down enamel over time.
2. The problem with citrus flavors
Think about your favorite flavors of sparkling water. Are they just plain or do you usually reach for a lemon or a lime flavored can of fizz? Turns out that there's a definite range of acidity when it come to sparkling water, and flavoring it with citrus can increase that acidity and potentially cause more damage to your enamel.
Studies have shown teeth begin to demineralize slightly at a pH of around 5.5. This is subject to a lot of different variables, so don't panic if you've been drinking a bunch of seltzer lately!
However, adding any sort of citrus flavor is certainly going to increase the degree of acidity in your drink, therefore putting your enamel at greater risk.
3. The problem with added sugars
As discussed, enamel forms the front line defense of your teeth. Anything that threatens to wear it away can mean serious damage for your teeth.
That's where sugar comes in: although it's not itself as acidic as lemon or lime, it can feed bacteria in your mouth that will then create an acidic environment. This will result in wear to the enamel, and possibly cavities and tooth decay.
So when it comes to sparkling water, make sure you pay attention to labels. It may not always be clear which brands are just carbonated water, and which brands include sugar to make the drink more enticing. Before you purchase, read the ingredients label and make sure no sugars were added.
What's the solution?
- If you're going to enjoy the occasional seltzer, try to keep it localized to just one time of the day. Drinking seltzer all day long exposes your enamel to low doses of acidity all day long.
- There's nothing better for your teeth than plain water!
- Make sure you prioritize dental hygiene. If you do, for example, drink a sugary bubbly drink, make sure you follow proper precautions by brushing and flossing thoroughly afterwards so that the sugars don't have time to react with bacteria in your mouth.
- Establish a tooth cleaning routine with your favorite dental team. You should go at least twice a year.
So when it comes to sparkling water and your enamel, it's not quite as bad as sugary drinks and candy. Drink it in moderation, always check labels for sugar, and try to avoid citrus flavors.
A quality dental team can answer all of your questions about teeth and make sure you smile is in the best condition possible. If you have any questions, or to schedule a consultation, contact Dr. Linger's dental practice today.



